RxC ProLOGS™
Product Lifecycle Optimization and Growth Strategy (An end-to-end Solution)
Product Lifecycle Optimization and Growth Strategy for Maximizing Asset Value
RxC ProLOGS service provides an end-to-end solution for brand life cycle management planning and development. RxC International's proprietary process incorporates in-depth market analysis, cross-functional client input, and industry best practices to produce actionable insights. This new service capability adds to RxC International's offerings as a premier life sciences management consulting firm.
The Critical Need for Drug Life Cycle Planning...
Life cycle planning is critical to maximizing an asset’s lifetime value. Patents generally provide 20-years of protection on drugs. However, by the time sufficient data is generated to show clinical proof of concept, the patent holders have already lost several years of protection. To maximize the remaining time while the drug is still under patent, biopharma companies must start life cycle planning initiatives as early as possible with a clear focus on addressing the needs of patients, providers, and payers.
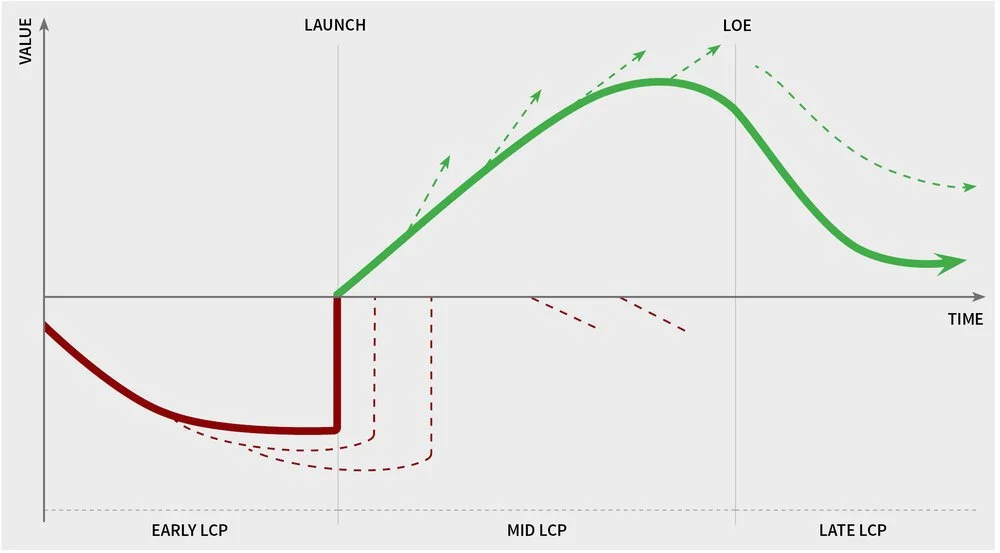
Life Cycle Planning Stages
Every drug goes through three phases: 1) clinical development, 2)commercialization during the exclusivity period, and 3) commercialization after the exclusivity period. These three stages present distinct life cycle opportunities:
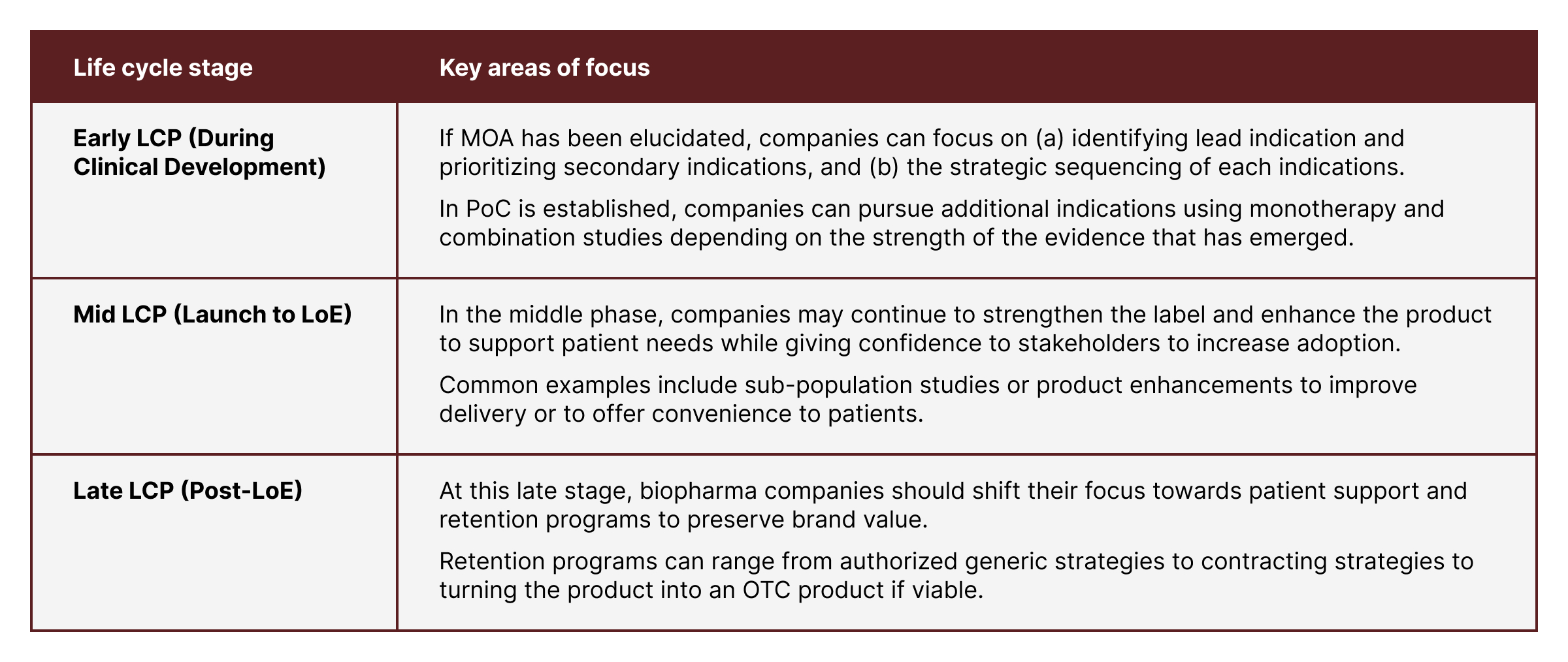
Early life cycle planning efforts start when the drugs are in clinical development
Mid-life cycle planning begins when the drugs are commercialized
Late life cycle planning revolves around the concept of maximizing the remaining value of the drug after patent expiration
The specific opportunities biopharma companies pursue under each stage can be markedly different, as highlighted here.
Introducing ProLOGS™ Solution
The ProLOGS™ framework (product lifecycle optimization and growth strategy) is designed to help biopharma companies develop and optimize plans for early, mid-, or late-stage life cycle planning to grow the product. ProLOGS™ is built based on the following guiding principles:
Patient-centricity is key to uncovering unmet needs along the patient journey
Translating these unmet needs into evidence-based solutions requires innovation
Life cycle opportunities must be differentiated leading to competitive advantage
Value imperative must be clear to patients, providers, payers and policymakers
Commercial and market access pathways must be clarified to avoid market surprises
Life cycle opportunities create incremental value as part of lifetime value maximization
RxC’s institutionalized approach leverages extensive experience to help clients develop life cycle applications efficiently and effectively, whatever the stage of the product life cycle. Successful life cycle programs will lead to competitive advantage and value creation.
How is ProLOGS™ Applied?
ProLOGS™ provides biopharma companies with a systematic approach to develop early, mid or late-stage life cycle plans. We collaborate with the cross-functional teams to develop life cycle opportunities that combine best scientific approaches and market-driven solutions.
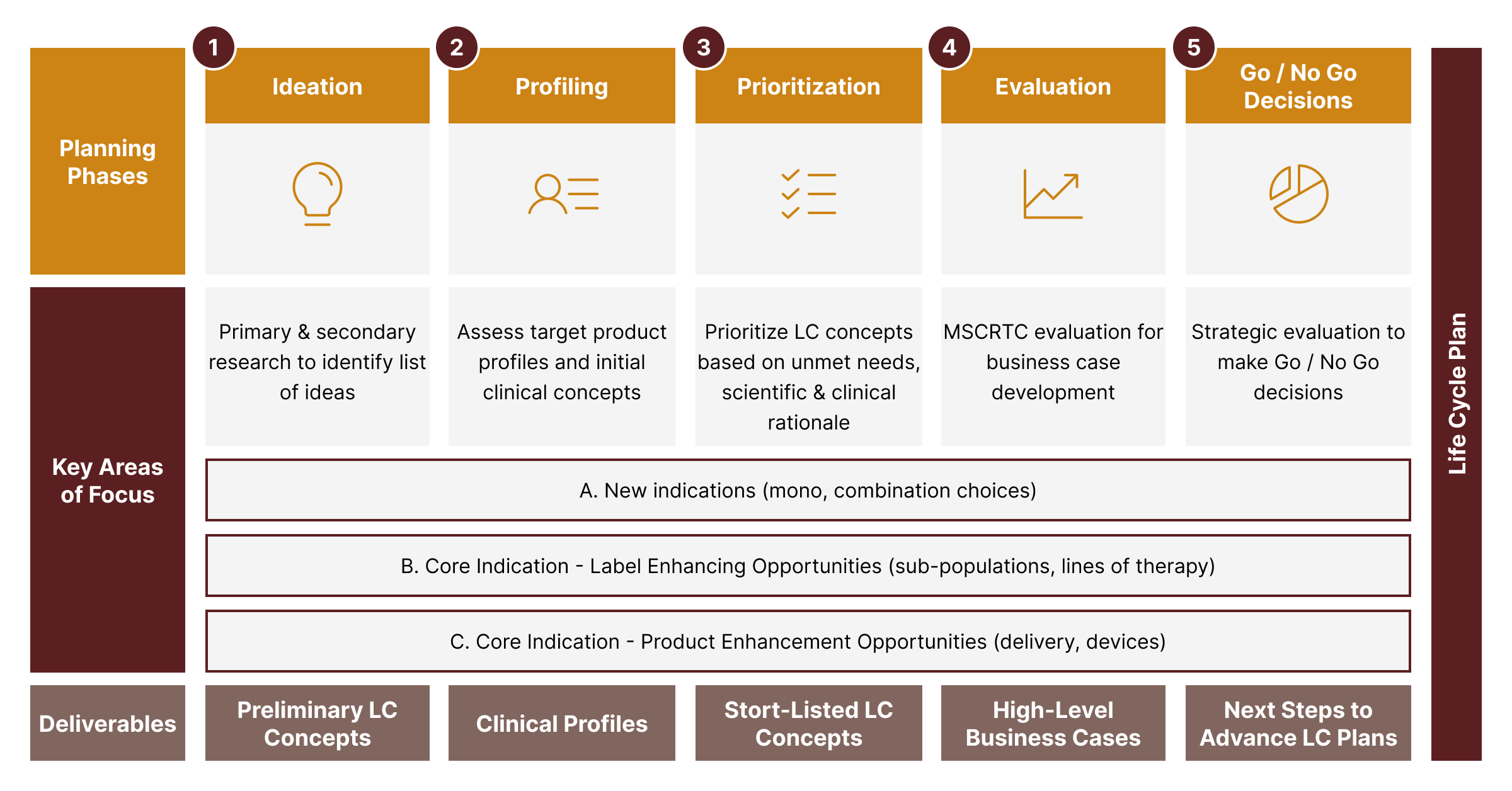
During a ProLOGS™ implementation, we will take clients through a proprietary five-step approach as outlined here.
In the Ideation Phase, we leverage primary and secondary research data to facilitate cross-functional working sessions to generate potential life cycle concepts.
In the Profiling Phase, we evaluate competing life cycle ideas to see which ones meet the basic criteria (e.g., strategic fit, unmet needs addressed, value creation) before advancing to the next stage.
In the Prioritization Phase, we apply the relative attractiveness criteria to prioritize and select a few viable opportunities for in-depth evaluation.
In the Evaluation Phase, we critically assess the opportunities that have been short-listed by using cross-functional input and external market research to quantify the potential value from a financial and strategic viewpoint. We also use sophisticated approaches to assess the probability of technical and regulatory success.
In the Decision-Making Phase, we guide management teams on how best to select the most attractive opportunities and develop roadmaps to help teams to advance them.
Best Practices Around Life Cycle Planning
The ProLOGS™ suite of solutions has been applied extensively for products in different stages of their development and commercialization. Through our experience, we have outlined five key factors that are critical to identifying and implementing high-impact life cycle opportunities. The objective methodology used in RxC ProLOGS™ considers the critical attributes of successful life cycle planning while customizing the criteria to meet each company’s objectives.
Accounting for product life-time value implications
Companies investigating potential life cycle options early in a product’s development have greater flexibility to explore the most comprehensive strategy and longer runway to execute this strategy. The pre-clinical stage, when planning teams are assessing potential lead indications and can also sequence follow-on indications, is the best time to begin life cycle planning since the company can plan its most impactful launch and also be prepared to initiate subsequent development efforts.
Building an evaluation framework for strategic and operational decisions
An objective, measurable, and market-tested framework helps drive more strategic and operationally successful life cycle planning decisions. we utilize our standardized criteria in this regard, addressing key scientific, clinical development, regulatory, intellectual property, technical operations, and commercial factors to determine which life cycle concepts warrant further exploration.
Taking a cross-functional approach to evaluating life cycle opportunities
Having the right cross-functional team—including representatives from the clinical, medical, IP / legal, manufacturing, regulatory, commercial and other (as needed) functions within the company—is vital to a life cycle planning process. Not only do these team members provide timely input to help generate innovative and robust ideas, but the early and continued buy-ins from such key stakeholders fosters broader organizational support that will help these life cycle strategies to be successfully developed and launched.
Using objective criteria to prioritize opportunities
An objective set of criteria must be used to prioritize life cycle management opportunities. Besides checking for how an individual life cycle plan aligns with corporate objectives and strategies, a scoring system that generates weight average scores can help evaluate the relative attractiveness of each plan—not only at the outset but along its critical path, setting up milestones and a roadmap that can be followed over the course of its implementation.
Developing business cases for making investment decisions
Once specific high-priority life cycle opportunities have been selected, the cross-functional team placed in charge must develop business cases to gain buy-in from key stakeholders within the organization. Specific attention must be paid to provide deeper insight into each functional element and present investment rationale that will form the basis for senior management decisions. Once the plans are approved, the life cycle team must continue to work with expanded cross-functional teams to execute according to plan.
Life cycle planning requires taking a strategic approach towards value creation that corresponds to the product’s development stage. A disciplined process allows companies to develop effective strategies while remaining responsive to key corporate objectives, as well as market and competitive developments and other unforeseeable factors.
The RxC Advantage
RxC International has extensive experience developing, evaluating, and implementing life cycle management plans both as pharmaceutical executives and industry consultants. Our team’s unique combination of strategic and operational expertise enables us to capture maximum value across the product life cycle. We have successfully completed numerous engagements and created significant value for clients.
Latest Publications on Life Cycle Planning
Clients and Case Studies
Check out examples of product commercialization work that our team has successfully completed for leading pharma and biotech companies.
RxC International has worked with a number of leading biopharma companies on commercialization initiatives. Our clients range from Fortune 100 companies to small cap companies in the life sciences sector.
RxC has worked with clients from around the world to successfully develop and commercialize dozens of products. Below is a representative list of projects to which we have applied our frameworks.